First-in-Class Drugs for Cancers
Mechanism of Action
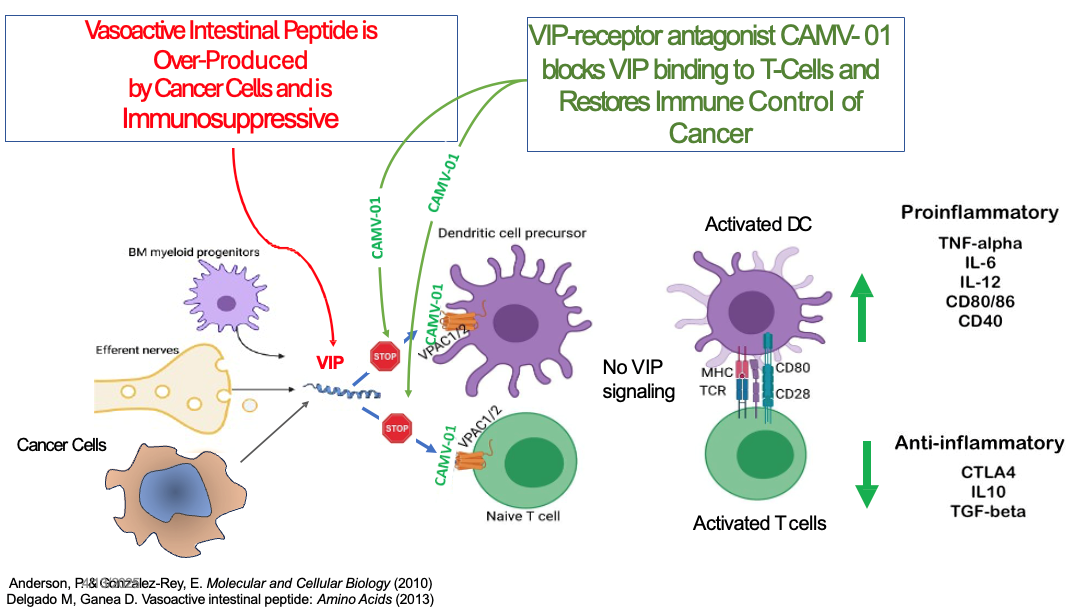
Cancer cells overproduce vasoactive intestinal peptide that suppresses the function of cytotoxic T-cells and prevents them from attacking tumor cells.
VIP binds to the VPAC1 receptors on cytotoxic T-cells thereby inhibiting tumor-killing signals.
Rationale for Targeting
VIP Receptors in Cancer
- Cancer cells over-express VIP creating immunosuppression that limits anti-cancer immunity.
- Activated T-cells up-regulate VIP-receptors.
- VIP is a conserved pathway in evolution. Cambium's VIP-receptor antagonists activate human T-cells and mouse T-cells.
- Cambium’s VIP-receptor antagonist enhances immunological attack on cancer in multiple cancer models.
- No toxicity of the CAMV-01, VIP-receptor antagonist, has been observed.
CAMV-01 potentiates the activation of human T-cells

Target Validation

Predicted binding affinities of novel VIP-receptor antagonists to human VIP-receptors correlate with the survival of leukemic mice treated with these drugs.
